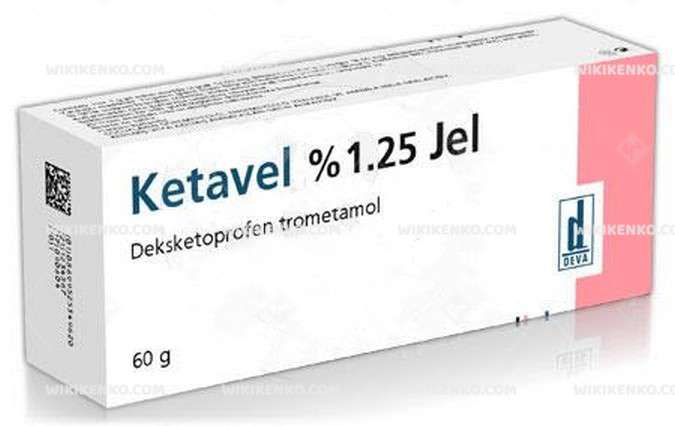
Ketavel Gel
Ketavel, a medication featuring ketoprofen, a prominent member of the non-steroidal anti-inflammatory drugs (NSAIDs) group, has emerged as a vital player in the realm of pain management. This comprehensive guide delves into the intricacies, unraveling its diverse applications and ensuring you’re well-informed.
| Dosage form | |
|---|---|
| Pack size | |
| Potency | %1.25 60G |
| Manufacturer | |
| Origin | |
| Generic Name (Ingredient) | 1 G Gel Contains 18.5 Mg Dexketoprofen Trometamol Equivalent To 12.5 Mg Dexketoprofen. |
Assuming your emergency circumstances for this product, visit Urgent Quotation page. Besides, for any pharmaceutical questions, please ask us in the comments section.
Description
Ketavel dons many hats, thanks to its chief component, ketoprofen. It’s available in various forms, including gel, tablet, and ampoule, providing flexibility for different needs. The primary objective is the reduction of pain and inflammation, making it a valuable asset in the medical world.
Applications of Ketavel:
Ketavel boasts a broad spectrum of applications, from addressing temporary pain conditions to managing chronic ailments. Here are some scenarios where Ketavel shines:
Temporary Pain Conditions
- Headaches
- Joint pain
- Migraines
- Toothaches
- Menstrual pain
Chronic Pain and Arthritis in Rheumatic Joint Diseases
- Sprains, strains, and muscle stretches
- Back pain
- Toothaches
- Menstrual cramps (dysmenorrhea)
- Headaches
- Migraines
- Rheumatoid arthritis
- Degenerative joint disease (osteoarthritis)
- Ankylosing spondylitis
- Bursitis and tendinitis
Dosage
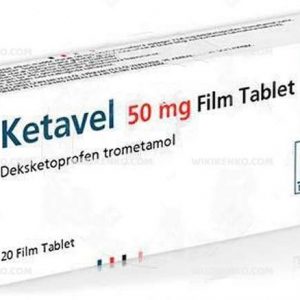
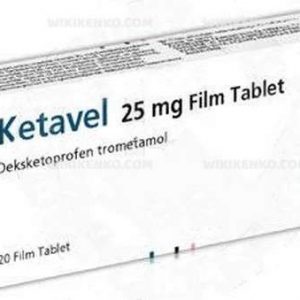
Ketavel offers various dosage options, depending on the form you choose. For instance, there are 25 mg and 50 mg tablets available. To optimize its benefits, meticulous adherence to your doctor’s or pharmacist’s instructions is crucial.
Storage
Maintaining the efficacy of Ketavel is straightforward. Store it at room temperature, between 20°C and 25°C. Avoid exposing it to damp or hot environments, such as bathroom or toilet cabinets.
Precautions
Ketavel may not be a one-size-fits-all solution. Before embarking on a Ketavel regimen, consult your healthcare provider if you fall into any of the following categories:
- Over 65 years old
- Suffer from heart, liver, kidney, blood pressure, circulation, or intestinal issues
- Previously experienced allergic reactions to ketoprofen or other medications
- Exhibit allergies to aspirin or other NSAIDs like ibuprofen or naproxen
- Have a history of asthma, runny nose, skin swelling (angioedema), or skin redness following NSAID consumption
- Grapple with stomach ulcers, stomach or intestinal bleeding, or a history of stomach wounds
- Battle high blood pressure
- Live with Crohn’s disease or ulcerative colitis
- Struggle with blood clotting disorders
- Have lupus
- Are trying to conceive, are pregnant, or are breastfeeding
It is not recommended for use by children under 18 years old without a doctor’s recommendation.
Conclusion
In conclusion, Ketavel, featuring ketoprofen, stands as a formidable contender in the battle against pain and inflammation. Its multifaceted applications cater to various temporary and chronic pain conditions. However, its safety and efficacy are best explored under the guidance of a healthcare professional. The potential for side effects exists, and understanding its interactions with other medications is paramount.
When considering it during pregnancy, breastfeeding, or for long-term pain relief, the counsel of medical experts is indispensable. As you navigate the path to pain relief, remember that your health and well-being deserve meticulous attention and a personalized approach. Ketavel, with its vast potential, may just be the solution you seek, provided you tread the path with knowledge and care.
Use the form below to report an error
Please answer the questions as thoroughly and accurately as possible. Your answers will help us better understand what kind of mistakes happen, why and where they happen, and in the end the purpose is to build a better archive to guide researchers and professionals around the world.
The information on this page is not intended to be a substitute for professional medical advice, diagnosis, or treatment. always seek the advice for your physician or another qualified health provider with any questions you may have regarding a medical condition. Always remember to
- Ask your own doctor for medical advice.
- Names, brands, and dosage may differ between countries.
- When not feeling well, or experiencing side effects always contact your own doctor.
Cyberchondria
The truth is that when we’re sick, or worried about getting sick, the internet won’t help.
According to Wikipedia, cyberchondria is a mental disorder consisting in the desire to independently make a diagnosis based on the symptoms of diseases described on Internet sites.
Why you can't look for symptoms on the Internet
If diagnoses could be made simply from a textbook or an article on a website, we would all be doctors and treat ourselves. Nothing can replace the experience and knowledge of specially trained people. As in any field, in medicine there are unscrupulous specialists, differences of opinion, inaccurate diagnoses and incorrect test results.


Reviews
There are no reviews yet.