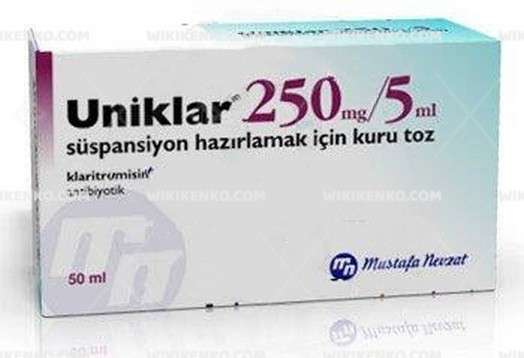
Uniklar Suspension Hazirlamak Icin Kuru Powder 125 250 Mg/5Ml
Uniklar Suspension Hazirlamak Icin Kuru Powder 125 250 Mg/5Ml, a pharmaceutical marvel, beckons as a valuable addition to the medical landscape. Manufactured by the esteemed Mustafa Nevzat in Turkey, this product boasts distinctive characteristics that warrant exploration.
| Dosage form | |
|---|---|
| Pack size | |
| Potency | 250 Mg/5Ml 50Ml |
| Manufacturer | |
| Origin | |
| Generic Name (Ingredient) | Clarithromycin 250 Mg |
Assuming your emergency circumstances for this product, visit Urgent Quotation page. Besides, for any pharmaceutical questions, please ask us in the comments section.
Description
Uniklar Suspension Ingredients
At its core lies the potent generic name, Clarithromycin. Each 5ml of this suspension embraces the therapeutic prowess of 250mg of Clarithromycin. Let’s delve deeper into the world of Clarithromycin and its multifaceted applications.
Clarithromycin: Unraveling Its Significance
Clarithromycin, the cornerstone of Uniklar Suspension Hazirlamak Icin Kuru Powder 125 250 Mg/5Ml, stands as a formidable macrolide antibiotic. Its mission is to wage war against bacterial intruders within your body, showcasing its effectiveness in combating a spectrum of infections.
Applications
Clarithromycin’s versatility shines through in its ability to address a myriad of bacterial infections that afflict the skin and respiratory system. Its battlefield includes strep throat, pneumonia, skin infections, H. pylori infection, Lyme disease, and more. Furthermore, it plays a pivotal role in the treatment of Helicobacter pylori, a bacterium notorious for its role in causing stomach ulcers.
Administration
The administration of Clarithromycin is user-friendly, allowing patients to choose between the convenience of pills or the flexibility of a liquid formulation.
Side Effects
In the pursuit of effective treatment, it is essential to be aware of potential side effects. Common occurrences include nausea, vomiting, headaches, and diarrhea. Fortunately, severe allergic reactions are a rarity. However, it is prudent to exercise caution if considering Clarithromycin during pregnancy, as it may pose risks.
Interactions
Clarithromycin’s interaction with other drugs demands attention. Several medications should not be combined with Clarithromycin, necessitating a potential adjustment to your treatment plan. Drugs like cisapride, pimozide, lomitapide, lovastatin, simvastatin, ergotamine, and dihydroergotamine fall into this category. Collaborate closely with your healthcare provider to ensure a harmonious treatment regimen.
Packaging and Availability
Uniklar Suspension Hazirlamak Icin Kuru Powder 125 250 Mg/5Ml is conveniently packaged in bottles, with a potency of 250 Mg/5Ml and a 50ml volume. To ascertain pricing and availability, login may be required. In urgent situations, the Urgent Quotation page offers a viable solution.
The Manufacturer
The custodian of this pharmaceutical gem is Mustafa Nevzat, a distinguished pharmaceutical company hailing from Turkey. With a commitment to quality and innovation, they stand as a symbol of trust in the medical realm.
Conclusion
In conclusion, Uniklar Suspension Hazirlamak Icin Kuru Powder 125 250 Mg/5Ml, fortified with the prowess of Clarithromycin, emerges as a valuable ally in the fight against a spectrum of bacterial infections. Its versatility, ease of administration, and cautious considerations make it a noteworthy choice. However, it is imperative to make all decisions regarding its use under the expert guidance of a healthcare professional. For comprehensive medical advice, always consult with a healthcare provider.
Product Details
| Attribute | Specification |
|---|---|
| Generic Name | Clarithromycin |
| Suspension Volume | 5ml |
| Clarithromycin Concentration | 250mg/5ml |
| Manufacturer | Mustafa Nevzat, Turkey |
| Availability | Login may be required for pricing |
| Packaging | Bottle |
| Pack Size | 1 |
Use the form below to report an error
Please answer the questions as thoroughly and accurately as possible. Your answers will help us better understand what kind of mistakes happen, why and where they happen, and in the end the purpose is to build a better archive to guide researchers and professionals around the world.
The information on this page is not intended to be a substitute for professional medical advice, diagnosis, or treatment. always seek the advice for your physician or another qualified health provider with any questions you may have regarding a medical condition. Always remember to
- Ask your own doctor for medical advice.
- Names, brands, and dosage may differ between countries.
- When not feeling well, or experiencing side effects always contact your own doctor.
Cyberchondria
The truth is that when we’re sick, or worried about getting sick, the internet won’t help.
According to Wikipedia, cyberchondria is a mental disorder consisting in the desire to independently make a diagnosis based on the symptoms of diseases described on Internet sites.
Why you can't look for symptoms on the Internet
If diagnoses could be made simply from a textbook or an article on a website, we would all be doctors and treat ourselves. Nothing can replace the experience and knowledge of specially trained people. As in any field, in medicine there are unscrupulous specialists, differences of opinion, inaccurate diagnoses and incorrect test results.


Reviews
There are no reviews yet.