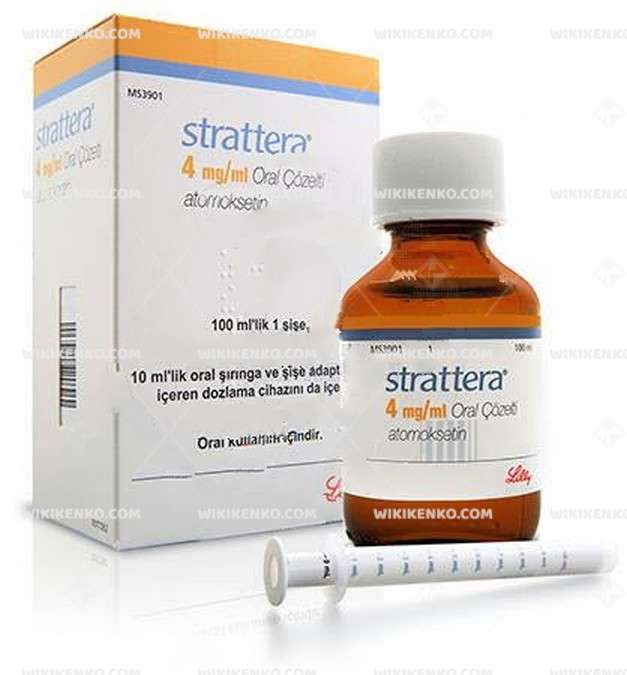
Strattera Oral Solution
Strattera Oral Solution, also known under its generic name atomoxetine, serves as a medication designated for the treatment of attention deficit hyperactivity disorder (ADHD). This article aims to provide an in-depth exploration of Strattera, encompassing its therapeutic applications, dosing regimens, potential adverse effects, and precautionary measures.
| Dosage form | |
|---|---|
| Pack size | |
| Potency | 4 Mg/Ml 100Ml |
| Manufacturer | |
| Origin | |
| Generic Name (Ingredient) | Each Capsule Contains Atomoxetine Hydrochloride Equivalent To 100 Mg Atomoxetine. |
Assuming your emergency circumstances for this product, visit Urgent Quotation page. Besides, for any pharmaceutical questions, please ask us in the comments section.
Description
Strattera constitutes an oral solution incorporating the active component atomoxetine hydrochloride. Classified as a member of the selective noradrenaline re-uptake inhibitors, Strattera Oral Solution presents a non-stimulant alternative for addressing ADHD manifestations, distinct from traditional stimulant medications.
Clinical Applications of Strattera Oral Solution
Strattera Oral Solution is indicated for:
- Children aged 6 years and older
- Adolescents
- Adults
It forms an integral facet of a comprehensive therapeutic regimen, often complemented by adjunctive counseling and behavioral modalities.
Warnings and Precautions
- Strattera Oral Solution usage is contraindicated in the presence of:
- Narrow-angle glaucoma
- Adrenal gland neoplasms
- Severe cardiovascular pathologies
- Pheochromocytoma
- Caution must be exercised in patients with a history of recent MAO inhibitor intake.
Potential Risks
- Strattera Oral Solution has been correlated with:
- Cerebrovascular incidents
- Cardiovascular morbidity
- Instances of sudden fatality in predisposed individuals
- Certain pediatric populations may exhibit emergent or aggravated psychotic presentations.
- Stringent monitoring protocols are imperative.
Dosage and Administration Guideines
- Strict adherence to healthcare provider directives is paramount.
- Dosage titration is contingent upon age, weight, and individual responsiveness.
- The oral solution formulation is dispensed in a child-safe container, accompanied by a calibrated dosing implement.
Side Effects
- Vigilance regarding potential adverse events is indispensable, encompassing:
- Psychotic episodes
- Suicidal ideation
- Timely reporting of mood fluctuations necessitates immediate intervention.
Considerations During Pregnancy and Breastfeeding
- Consultative discourse with healthcare professionals is imperative for gravid or prospective gestational individuals.
- The teratogenic potential of Strattera necessitates meticulous antenatal monitoring.
- Deliberation regarding breastfeeding implications mandates comprehensive clinical consultation.
Conclusion
In conclusion, Strattera Oral Solution delineates a noteworthy therapeutic paradigm within the domain of ADHD management. Diligent consultation with healthcare providers, coupled with meticulous surveillance, constitutes cardinal principles for optimizing therapeutic efficacy.
It is incumbent upon individuals to assimilate the elucidated insights as constituting a generic exposition. Tailored counsel predicated on individual exigencies mandates recourse to personalized healthcare professional consultation.
Please note that the content provided herein is intended for educational and informational purposes exclusively. Readers are encouraged to confer with their healthcare providers for contextually pertinent guidance and counsel.
Manufacturers of Generic Atomoxetine Hydrochloride Capsules
| Manufacturer | Approval Date | Strengths |
|---|---|---|
| APOTEX | May 30, 2017 | 10MG, 18MG, 25MG, 40MG, 60MG, 80MG, 100MG |
| AUROBINDO PHARMA | May 30, 2017 | 10MG, 18MG, 25MG, 40MG, 60MG, 80MG, 100MG |
| DR REDDYS | Feb 23, 2018 | 10MG, 18MG, 25MG, 40MG, 60MG, 80MG, 100MG |
| GLENMARK PHARMS LTD | May 30, 2017 | 10MG, 18MG, 25MG, 40MG, 60MG, 80MG, 100MG |
| HETERO LABS LTD V | Mar 11, 2021 | 10MG, 18MG, 25MG, 40MG, 60MG, 80MG, 100MG |
| TEVA PHARMS USA | May 30, 2017 | 10MG, 18MG, 25MG, 40MG, 60MG, 80MG, 100MG |
| ZYDUS PHARMS USA INC | Sep 17, 2010 | 18MG, 25MG, 40MG, 60MG, 80MG, 100MG |
| ZYDUS PHARMS USA INC | Apr 5, 2023 | 10MG |
Use the form below to report an error
Please answer the questions as thoroughly and accurately as possible. Your answers will help us better understand what kind of mistakes happen, why and where they happen, and in the end the purpose is to build a better archive to guide researchers and professionals around the world.
The information on this page is not intended to be a substitute for professional medical advice, diagnosis, or treatment. always seek the advice for your physician or another qualified health provider with any questions you may have regarding a medical condition. Always remember to
- Ask your own doctor for medical advice.
- Names, brands, and dosage may differ between countries.
- When not feeling well, or experiencing side effects always contact your own doctor.
Cyberchondria
The truth is that when we’re sick, or worried about getting sick, the internet won’t help.
According to Wikipedia, cyberchondria is a mental disorder consisting in the desire to independently make a diagnosis based on the symptoms of diseases described on Internet sites.
Why you can't look for symptoms on the Internet
If diagnoses could be made simply from a textbook or an article on a website, we would all be doctors and treat ourselves. Nothing can replace the experience and knowledge of specially trained people. As in any field, in medicine there are unscrupulous specialists, differences of opinion, inaccurate diagnoses and incorrect test results.


Reviews
There are no reviews yet.