Description
What Is Fraxiparine?
Fraxiparine Subkutan, with its generic name nadroparin, falls under the category of anticoagulants or antithrombotic agents. These medications function by reducing the clotting propensity of blood, thereby mitigating the risk of harmful clot formation within blood vessels.
Indications and Uses
Deep Vein Thrombosis (DVT) Prevention and Treatment
Deep Vein Thrombosis (DVT) arises when blood clots form within the deep veins of the legs. If left unaddressed, these clots can migrate to the lungs, instigating pulmonary embolism, a grave medical condition. Fraxiparine proves instrumental in averting both DVT and pulmonary embolism.
Surgical Procedures
Fraxiparine finds extensive utility in patients undergoing various surgical interventions, including general and orthopedic surgeries like hip or knee replacements. It is also beneficial for individuals immobilized or hospitalized due to severe ailments such as heart failure, profound chest infections, or respiratory failure.
Hemodialysis
Patients undergoing hemodialysis rely on Fraxiparine to forestall clot formation during the procedure.
Unstable Angina and Heart Attack
Fraxiparine, or nadroparin, serves as a therapeutic agent for addressing unstable angina and supporting individuals who have survived a heart attack.
Dosage and Administration
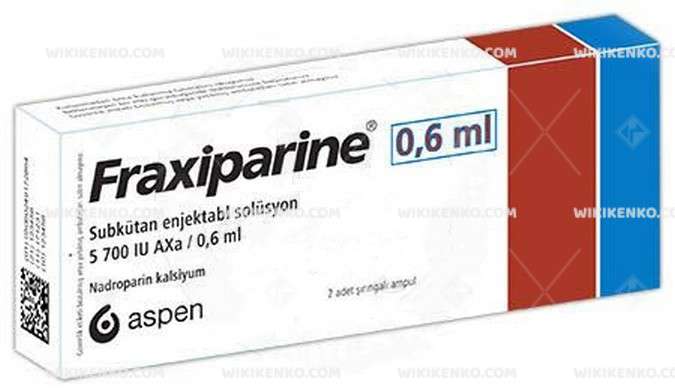
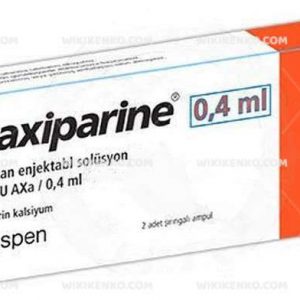
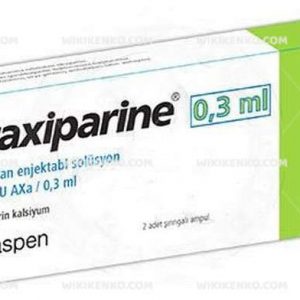
Fraxiparine is conveniently available in prefilled syringes, offering varied IU (anti-Xa) concentrations to suit individual requirements:
| Dosage | IU (Anti-Xa) |
|---|---|
| 0.3 mL | 2,850 |
| 0.4 mL | 3,800 |
| 0.6 mL | 5,700 |
| 1 mL | 9,500 |
Important Considerations
- Always adhere strictly to the prescribed dosage and administration schedule as directed by your healthcare provider.
- Discontinuing Fraxiparine usage abruptly without medical consultation is ill-advised.
- Refrain from sharing Fraxiparine with others unless explicitly instructed by a qualified healthcare professional.
Storage Guidelines
Fraxiparine Subkutan Injection 5700 IU/0.6 mL should be stored under the following conditions:
- Temperature: Maintain Fraxiparine below 25°C (77°F).
- Freezing: Avoid subjecting the medication to freezing temperatures.
- Refrigeration: Steer clear of refrigerating Fraxiparine, as cold injections can elicit discomfort.
- Child Safety: Safeguard the medication out of children’s reach, ideally in a locked cupboard positioned at least one-and-a-half meters above ground level.
Storage Duration of Opened Syringe
Upon opening a syringe of Fraxiparine Subkutan Injection 5700 IU/0.6 mL, it is crucial to adhere to specific storage guidelines to maintain its efficacy:
- Shelf-Life After Opening: The European Medicines Agency (EMA) stipulates that if no notable alterations are observed within three months of open-dish storage, no specific in-use shelf life is mandated. However, any discernible changes necessitate further in-use studies for determining shelf life.
- Storage Recommendations: Ensure storage below 25°C (77°F), avoiding freezing and refrigeration. Store the medication out of children’s reach in a secure location.
Signs of Allergic Reaction
Fraxiparine Subkutan Injection 5700 IU/0.6 mL may provoke allergic reactions in susceptible individuals, characterized by manifestations such as:
- Shortness of Breath
- Swelling of Face, Lips, or Tongue
- Skin Rash or Itching
- Severe Headache, Coordination Difficulties, or Confusion
- Visual Disturbances or Weakness
Prompt medical attention is warranted upon experiencing any of these symptoms.
Fraxiparine Subkutan Side Effects
Common side effects associated with Fraxiparine Subkutan Injection 5700 IU/0.6 mL may include:
- Injection Site Reactions
- Unusual Bleeding or Bruising
- Dark Red Spots in the Mouth
- Other Adverse Reactions
Individual responses may vary, and consultation with healthcare providers is recommended in case of any concerns or adverse reactions.
Administration Timing
While Fraxiparine administration typically entails a once-daily injection, the optimal timing may vary based on individual circumstances and healthcare provider recommendations. Morning administration is commonly advised, although nighttime dosing may also offer potential benefits, subject to individual considerations.
Conclusion
Fraxiparine Subkutan Injection 5700 IU/0.6 mL stands as a cornerstone in the management of blood clot-related disorders, offering efficacy and convenience. However, personalized medical guidance is indispensable for optimal therapeutic outcomes. Always prioritize consultation with your healthcare provider to address specific concerns and ensure safe and effective usage of this medication.









Reviews
There are no reviews yet.