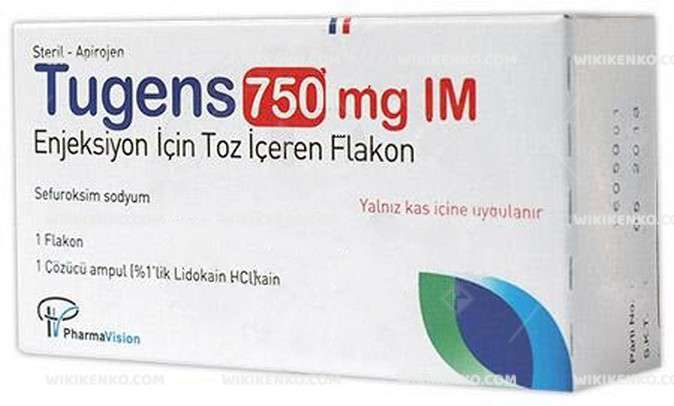
Tugens Im Injection Icin Powder Iceren Vial 750 Mg
Tugens IM Injection Icin Powder Iceren Vial 750 Mg, manufactured by Pharmavision in Turkey, offers a unique pharmaceutical solution encapsulated within a vial.
| Dosage form | |
|---|---|
| Pack size | |
| Potency | 750 Mg |
| Manufacturer | |
| Origin | |
| Generic Name (Ingredient) | In A Vial; Cefuroxime Sodium 788, 81 Mg (Cefuroxime Equivalent Of 750 Mg) A Solvent In The Ampoule (3 Ml); Lidocaine Hirdochloride 30 Mg (1%) |
Assuming your emergency circumstances for this product, visit Urgent Quotation page. Besides, for any pharmaceutical questions, please ask us in the comments section.
Description
Ingredients
The core of Tugens IM Injection lies in its composition, which includes Cefuroxime Sodium 788.81 Mg, equivalent to Cefuroxime 750 Mg, and Lidocaine Hydrochloride 30 Mg (1%) in a 3 mL ampoule.
Dosage Form and Quantity
Tugens IM Injection is packaged as a single vial, signifying its dosage form. Each pack of this medication encompasses one vial.
Origin
This medicinal enigma hails from the vibrant pharmaceutical landscape of Turkey.
Limited Information
Despite our meticulous investigation, detailed information about Tugens IM Injection remains elusive within Turkish sources. To acquire a comprehensive understanding, we strongly recommend consulting a healthcare professional or pharmacist.
Side Effects
Within Tugens IM Injection, two active ingredients coexist, each with its potential side effects:
Cefuroxime Sodium: The Potential Gambit
Cefuroxime Sodium, a second-generation cephalosporin antibiotic, is adept at combating various bacterial infections. However, like any formidable weapon, it bears its own set of potential side effects.
Common Side Effects:
- Diarrhea
- Fever
- General discomfort or malaise
- Headache
- Vaginal or genital itching
- Pain during sexual intercourse
- Rigidity
- Sweating
- Thick, odorless or mildly odorous vaginal discharge
Less Common Side Effects:
- Black, tarry stools
- Chest pain
- Cough
- Loose stools
- Painful or challenging urination
- Shortness of breath
- Sore throat
- Sores, ulcers, or white mouth/lip spots
- Swollen glands
- Unusual bleeding or bruising
- Unusual fatigue or weakness
Rare Side Effects:
- Back, leg, or stomach pains
- Bladder discomfort
- Bleeding gums
- Cloudy or bloody urine
- Body aches or pain
- Burning sensation during urination
- Dark urine
- Difficulty in breathing
- Ear congestion
Lidocaine Hydrochloride: The Tame Anesthetic
Lidocaine Hydrochloride, a mild local anesthetic, occasionally contributes the following side effects:
Common Side Effects:
- Low blood pressure (hypotension)
- Swelling (edema)
- Redness at the injection site
- Small red or purple skin spots
- Skin irritation
Less Common Side Effects:
- Bluish lips, fingernails, or palms
- Blurred or double vision
- Chest discomfort or pain
- Cold, clammy, pale skin
- Continuous ringing or unexplained noise in the ears
- Breathing difficulties
- Difficulty swallowing
- Dizziness or lightheadedness
Please note that this list is not exhaustive, and individual experiences may vary. It’s crucial to remember that the mere presence of a side effect here does not imply that every user will encounter it. If any alterations in your health come to light while on this medication, promptly seek counsel from a healthcare professional.
Guidance on Usage: Tugens IM Injection
Specific usage instructions for Tugens IM Injection remain undisclosed. However, we offer a general outline for utilizing injectable medications in powdered form:
Reconstitution Protocol: Exercise unwavering aseptic technique during reconstitution. Utilize sterile needles and syringes to prepare the medication.
Administration: Following reconstitution, the medication typically finds its way into the patient’s system either intravenously (into a vein) or intramuscularly (into a muscle). The chosen administration method hinges on the specific medication and the patient’s unique medical circumstances.
Storage: After reconstitution, select medications necessitate immediate use, while others tolerate short-term storage under specific conditions. Protection from direct sunlight and avoidance of subjecting it to freezing temperatures are critical considerations.
Please note that these guidelines offer a general overview and may not apply universally. Always adhere to the specific instructions imparted by your healthcare provider or pharmacist when handling any medication. When questions or concerns arise regarding this medication’s use, a healthcare professional can offer the most accurate and current information.
Precautions
Guarding Your Health: Precautions for Tugens IM Injection Icin Powder Iceren Vial 750 Mg
While precise precautions for using Tugens IM Injection await revelation, we offer general safety measures for handling injectable medications in powdered form:
Reconstitution Protocol: Exercise unwavering aseptic technique during reconstitution. Utilize sterile needles and syringes to prepare the medication.
Professional Administration: Ensure that only a healthcare professional with the necessary medical support oversees the administration of this medication. Serious infusion-related reactions, potentially fatal, can manifest if not managed correctly.
Prudent Storage: Following reconstitution, some medications mandate immediate use, while others tolerate short-term storage under specific conditions. Shield them from direct sunlight and prevent freezing.
Hypersensitivity Caution: As with all beta-lactam antibacterial agents, severe and occasionally fatal hypersensitivity reactions have been reported in association with Cefuroxime Sodium. Should such reactions arise, immediate discontinuation of Cefuroxime Sodium treatment becomes imperative.
These precautions serve as general guidelines and may not universally apply. Always adhere meticulously to the specific directives provided by your healthcare provider or pharmacist when dealing with any medication.
Onset of Actions
The curtain of secrecy veils the onset of action for Tugens IM Injection Icin Powder Iceren Vial 750 Mg. However, it’s critical to comprehend that the onset of action for injectable medications is a variable enigma. Its unveiling relies on a multifaceted interplay of medication type, dosage, patient body mass, metabolic rate, and overall health.
For precision on this medication’s onset of action, your most reliable resource remains the counsel of a healthcare professional. They possess the expertise to furnish you with the most accurate and timely insights.
Cefuroxime Sodium Chronicle
Cefuroxime Sodium, a second-generation cephalosporin antibiotic, stands as a stalwart guardian against an array of bacterial infections. Its dominion extends over ailments such as pneumonia, meningitis, otitis media, sepsis, urinary tract infections, and Lyme disease. Administered via oral consumption or injection into veins or muscles, this antibiotic wages war against bacteria by inhibiting their cell wall formation, ultimately leading to their demise.
However, it’s imperative to remember that Cefuroxime Sodium’s prowess is confined to bacterial infections. It remains impotent in the face of viral adversaries, such as the common cold and flu. Misuse or unwarranted use of antibiotics can usher in diminished efficacy.
Conclusion
In our journey through the labyrinthine world of pharmaceuticals, we’ve illuminated the contours of Tugens IM Injection Icin Powder Iceren Vial 750 Mg. While some aspects remain shrouded in mystery, this guide has endeavored to provide clarity on its composition, potential side effects, usage guidelines, precautions, and the active ingredient, Cefuroxime Sodium.
In the realm of healthcare, knowledge serves as the compass, and healthcare professionals remain our guiding lights. For comprehensive information and guidance tailored to your specific needs, we urge you to seek the counsel of a healthcare professional or pharmacist. In their expertise, you’ll discover the answers you seek and the care you deserve.
Table: Tugens IM Injection Icin Powder Iceren Vial 750 Mg Overview
| Attribute | Details |
|---|---|
| Medication Name | Tugens IM Injection |
| Composition | Cefuroxime Sodium 788.81 Mg (equivalent to Cefuroxime 750 Mg), Lidocaine Hydrochloride 30 Mg (1%) |
| Dosage Form and Pack Size | Vial (One per pack) |
| Manufacturer | Pharmavision |
| Origin | Turkey |
| Common Side Effects (Cefuroxime Sodium) | Diarrhea, Fever, General discomfort, Headache, Itching of genital area, Pain during sexual intercourse, Rigidity, Sweating, Thick vaginal discharge |
| Less Common Side Effects (Cefuroxime Sodium) | Black, tarry stools, Chest pain, Cough, Loose stools, Painful urination, Shortness of breath, Sore throat, Sores in mouth, Swollen glands, Unusual bleeding, Unusual tiredness |
| Rare Side Effects (Cefuroxime Sodium) | Back, leg, or stomach pains, Bladder pain, Bleeding gums, Bloody or cloudy urine, Body aches, Burning during urination, Dark urine, Difficulty breathing, Ear congestion |
| Common Side Effects (Lidocaine Hydrochloride) | Low blood pressure (hypotension), Swelling (edema), Redness at injection site, Skin spots, Skin irritation |
| Less Common Side Effects (Lidocaine Hydrochloride) | Bluish lips/nails/palms, Blurred or double vision, Chest pain, Cold, clammy skin, Ear noise, Difficulty swallowing, Dizziness |
| Storage Requirements | Protect from direct sunlight, Do not freeze |
| Precautions | Follow aseptic technique during reconstitution, Administer under healthcare professional supervision, Be cautious of hypersensitivity reactions |
Note: This table provides an overview of key attributes and side effects. Consult a healthcare professional or pharmacist for comprehensive guidance and information specific to your situation.
Use the form below to report an error
Please answer the questions as thoroughly and accurately as possible. Your answers will help us better understand what kind of mistakes happen, why and where they happen, and in the end the purpose is to build a better archive to guide researchers and professionals around the world.
The information on this page is not intended to be a substitute for professional medical advice, diagnosis, or treatment. always seek the advice for your physician or another qualified health provider with any questions you may have regarding a medical condition. Always remember to
- Ask your own doctor for medical advice.
- Names, brands, and dosage may differ between countries.
- When not feeling well, or experiencing side effects always contact your own doctor.
Cyberchondria
The truth is that when we’re sick, or worried about getting sick, the internet won’t help.
According to Wikipedia, cyberchondria is a mental disorder consisting in the desire to independently make a diagnosis based on the symptoms of diseases described on Internet sites.
Why you can't look for symptoms on the Internet
If diagnoses could be made simply from a textbook or an article on a website, we would all be doctors and treat ourselves. Nothing can replace the experience and knowledge of specially trained people. As in any field, in medicine there are unscrupulous specialists, differences of opinion, inaccurate diagnoses and incorrect test results.


Reviews
There are no reviews yet.