Septonat Oral Spray
Active Item:
at 30 ml (1 bottle);
Chlorhexidine Gluconeate: 36 mg
Benzidamin HCl: 45 mg
Auxiliary Substance(s):
Sorbitol (70%): 6 g
Glycerine: 6 g
| Dosage form | |
|---|---|
| Pack size | |
| Potency | 30Ml |
| Manufacturer | |
| Origin | |
| Generic Name (Ingredient) | 30 Ml (1 Bottle); Chlorhexidine Gluconate 36 Mg Benzidamine Hcl 45 Mg |
Assuming your emergency circumstances for this product, visit Urgent Quotation page. Besides, for any pharmaceutical questions, please ask us in the comments section.
Description
PHARMACEUTICAL FORM
Oral spray
Clear, colorless solution.
Therapeutic indications
- By assisting antibacterial treatment suitable for angin, pharyngitis, laryngitis and tonsillitis,
- In oral cavity mucositis due to chemotherapy, radiotherapy or other physical causes (such as tracheal intubation),
- Aft ulcers, glossitis,
- Temporary relief of painful mouth and throat conditions after oral surgery and involving periodontal procedures,
- In the treatment and prevention of gingivitis,
- In the treatment of oral infections,
- In the improvement of oral complications,
- In the treatment of oral mucous inflammations (stomatitis),
- Prophylacticly in preventing the formation of dental plaques,
- Paradental diseases, paradentosis due to excessive strain,
- Conservative dental treatments and tooth extractions as an auxiliary treatment,
- It is used to ensure oral hygiene.
Poseology and application Method Posiology/application frequency and duration:
General dose of SEPTONAT in direct application to throat / inflamed area 5-10
1/2
is a spray. It is repeated every 1 -3 hours if necessary.
How to apply:
SEPTONATE is used without dilution. SEPTONATE should not be swallowed and removed from the mouth by spitting.
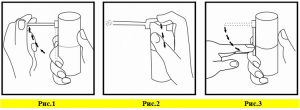
Before first use, the pumping button should be pressed several times, holding it in a remote direction from the hundred, until a regular spraying is obtained.
The mouth should be opened thoroughly, the spray nose inserted into the mouth, squeezed into the oral cavity. This process should be repeated at least 4 times in different regions.
After applying, it should be placed in the bottle box and stored upright. Chlorhexidine contained in SEPTONATE decreases plaque and gingivitis during treatment. Septonate should be kept in the mouth for at least 1 minute if it is used as an alternative to oral hygiene procedures.
It is convenient to brush the teeth before use to minimize the coloration caused by chlorhexid in SEPTONATE.
Additional information on specific populations:
Kidney/Liver failure:
In patients with severe renal disorder and severe liver disorder, it should be used with caution considering the possibility of systemic action (see Section 4.4).
Paediatric population:
in children over the age of 12, the spray is applied directly to the throat / inflamed area.
1/2
The general dose is 5 sprays. It is repeated every 1 -3 hours if necessary.
Septonat under the age of 12 due to insufficient number of clinical trials
not recommended in children
Geriatric population:
Geriatric patients can be given the same dose as adults.
Contraindications
Benzydamine and chlorhexidine and any of the substances in septonate
is contraindicated in those with hypersensitivity.
It should not be used during pregnancy and breastfeeding (see Section 4.6).
Special use warnings and precautions
- Used externally.
- SEPTONATE is not recommended in children under the age of 12 due to insufficient number of clinical trials.
- It is used only in the mouth, contact with the eyes and ears should be avoided. If it comes into contact with the eye, it should be thoroughly washed with plenty of water immediately.
- It can make recycled color changes in the mouth, on the tongue and tooth. To minimize coloration, it is convenient to brush the teeth before use.
- SEPTONATE should not be ingested and removed from the mouth by spitting. It is used without dilution.
- If sore throat is caused by bacterial infection or is seen with infection, antibacterial treatment may be considered in addition to septonate use.
- Since absorbed benzydamine and its metabolites are excreted in urine, the possibility of systemic effects should be considered in patients with severe renal disorder.
- Since absorbed benzydamine is highly metabolized in the liver, the possibility of systemic action should be considered in patients with severe liver disorders.
- Since this medical product contains sorbitol, patients with rare hereditary fructose intolerance should not use this drug.
Interactions with other medical products and other forms of interaction
- SEPTONATE has no known significant drug interaction. Chlorhexide, one of the active ingredients it contains, has a miscompass with certain substances.
- Chlorhexidine salts are insulated with soap and other anyonic compounds.
- Chlorhexidine salts are in use with cationic and nonionic surfacts, but when used together in high concentrations, the activity of chlorhexidine may decrease due to mycenic bonding.
- The solusity of chlorhexidine salts can be increased with frictions such as setrimid and lissapol NX.
- Chlorhexidine is inconsequeminated with anyonic polyelectryls such as arabic glue, sodium alginate, sodium carboxy methyl cellulose, starch and kitre glue, but also its effects are reduced with these substances.
- Chlorhexidine, brillant green, chloramphenicol, copper sulfate, florescein sodium, formaldehyde, silver nitrate, zinc sulfate are also insatiable.
- Chlorhexidine can collapse into insoluble salts as it interacts with Ca and Mg cations when diluted with hard water.
- Solutions of chlorhexidine salts combined with benzoates, bicarbonates, carbonates, borates, nitrates, phosphates, sulfates will form less salts if combined with less than 0.05%. Since setrimid increases the solusity of these salts, these collapses do not happen when combined with setrimid.
- Chlorhexidine is insatiable with gluconate, setrimide and benzalconium chloride. They increase the effect of bacteriids synergistically. Setrimid prevents the collapse of chlorhexid with hard water.
- Chlorhexidine and salts, excluding gluconate, are better soluble in alcohol than in water. Chlorhexidine gluconate solution can collapse when added to alcohol. The presence of ethanol in the formulation makes the solution more effective against Gram negative microorganisms. They can be adsorbed when filtering through cellulosic filters.
- No drug interaction with benzydamin has been reported.
- General advice on pregnancy and lactation
Pregnancy category : C
Women with the potential to have children/Contraception (Contraception)
SEPTONATE has no effect on contraception.
Gestation period
The use of SEPTONATE during pregnancy is contraindicated.
Studies on animals are inadequate in terms of effects on pregnancy and/or embryonal/fetal development and/or postpartum and/or postpartum development. The potential risk to humans is unknown.
Lactation period
No data is available in breastfeeding women. Therefore, its use in breastfeeding mothers is contraindicated.
Reproductive ability/Fertility
There are studies on reproduction and fertility with chlorhexidine gluconate.
On fertility in rats; again, no harmful effect on the fetus was seen in rats and rabbits.
Effects on vehicle and machine use
There is no negative effect on the use of tools and machines.
Undesirable effects
Unwanted effects are divided into frequency groups using the following classification.
Very common (>1/10); common (>1/100 to <1/10); unsymbi d’or (>1/1,000 to <1/100); sparse (>1/10,000 to <1/1,000); very rare (<1/10,000), unknown (unpredictable based on available data).
SEPTONATE is generally well tolerated and has very few side effects.
There are no serious side effects and adverse effects reported at the end of clinical trials.
More local side effects are observed. Systemic side effects are usually not seen and serious.
Immune system disorders:
Very rare: Allergic reaction, hypersensitivity and anaphylaxis
Nervous system disorders:
Very common: Temporary sensation reduction in the mouth Common: Feeling of stinging and burning in the mouth Unknown: Dizziness, headache, lethargy
Endocrine system diseases:
Very rare: Temporary swelling of the parotis gland
Respiratory, chest disorders and mediastinal diseases:
Very rare: Larynospasm, bronchospasm Unknown: Faringeal irritation, cough
Gastrointestinal disorders:
Common: Nausea, vomiting, gag Unknown: Dry mouth
Skin and subcutaneous tissue diseases:
Very rare: Skin reactions due to irritation, itching associated with rash, urticaria, photodermatitis, oral deskuamation
Other side effects such as local dryness or thirst, aching, a feeling of coolness in the mouth and changes in taste, staining of teeth and other oral surfaces, such as an increase in calculus formation, are generally less. Tooth staining is harmless and can be minimized by brushing teeth before application.
Overdose and treatment
Poisoning is not possible when the method of application of the active substance is taken into account.
However, if SEPTONATE is drunk by mistake, symptomatic treatment should be carried out. There is no specific antidote.
PHARMACOLOGICAL FEATURES
Pharmacodinamic properties
Pharmacotherapy group: Antiseptic (Topical Pharyngeal), Topical oral anti-inflammatory ATC code: A01AD02
Benzydamine is an anti-inflammatory analgesic agent not associated with the steroid group. Benzydamine is different from other non-steroidal anti-inflammatory agents in terms of base.
Benzydamine has a local anesthetic effect in the concentrations used in topical treatment. Analgesic activity of benzydaminin was more reported in models with experimental inflammation than non-inflammatory pain.
Like other non-steroidal anti-inflammatory agents, benzydamine inhibits prostaglandin biosynthesis under certain conditions. However, this feature has not been fully explained. The stabilizing effects on cellulose membranes can be attributed to the mechanism of action.
After normal topical administration of the drug, chlorhexidine has a bacteriocidal effect on the back of its prolonged bacteriostatic effect.
Chlorhexidine is a biguanid antiseptic and helps reduce the development of plaque and gingivitis when general oral hygiene is suspended. Tooth enamel hydroxyapatite has a strong structure with its affinity to oral structures containing tooth surface, bacterial and saliva proteins. Reduces dental plaque depositation of chlorhexidine and gingivada gingivit, which is characterized by redness, swelling or bleeding. Reduces the frequency of aft ulcers and increases the rate of recovery after periodontal surgery.
Chlorhexidine is effective in most gram(+) and gram(-) bacteria, yeast and some fungi and viruses. Delayed surface effect of chlorhexidine delays bacterial reproduction. It absorbs through the walls of microbial cells and causes membrane leakage.
Pharmacokinetic properties General features
Absorption:
Following the topical application of SEPTONATE, benzydamine is easily absorbed into the inflamed local mucosa, which will show its anti-inflammatory and local anesthetic effects.
Distribution:
About 30% of the chlorhexidine administered remains in the oral cavity and is slowly released into oral fluids for 24 hours.
Biotransformation:
The absorbed benzydamin metabolizes highly in the liver.
Elimination:
Absorbed benzydamine and its metabolites are excreted in urine.
Preclinical safety data
There are studies on reproduction and fertility with chlorhexidine gluconate. On fertility in rats; again, no harmful effect on the fetus was seen in rats and rabbits.
PHARMACEUTICAL PROPERTIES
List of auxiliary items
Glycerin Sorbitol (70%)
Hydroxysieline cellulose Strawberry aroma Pure water
Insperiences
Salts of chlorhexidine, it is insatiable with soap and other anyonic compounds, anyonic polyelectroids such as arabic glue, sodium alginate, sodium carboxy methyl cellulose, with relative and kitre glue, brillant green, chloramphenicol, copper sulfate, floressein sodium, formaldehyde, silver nitrate, zinc sulfate (See Section 4.5).
Shelf life
24 months
Special measures for storage
It should be stored at room temperature below 25°C.
It should be placed in the bottle box and stored upright.
Quality and content of the packaging
30 ml opaque PET bottle with oral applicator
Disposal of residual substances from human medical products and other special measures
Unused products or waste materials must be disposed of in accordance with the ‘Medical Waste Control Regulation’ and the ‘Packaging and Packaging Waste Control Regulations’.
LICENSE HOLDER
VEM Pharmaceuticals Inc. and Tic. Ltd. Sti.
Cinnah Cad. Yeşilyurt Sok. No: 3/2 Çankaya / Ankara Phone : (0312) 427 43 57-58
Fax : (0312) 427 43 59
LICENSE NUMBER(LARI)
241/71
FIRST LICENSE DATE/LICENSE RENEWAL DATE
First license date: 21.03.2012
Use the form below to report an error
Please answer the questions as thoroughly and accurately as possible. Your answers will help us better understand what kind of mistakes happen, why and where they happen, and in the end the purpose is to build a better archive to guide researchers and professionals around the world.
The information on this page is not intended to be a substitute for professional medical advice, diagnosis, or treatment. always seek the advice for your physician or another qualified health provider with any questions you may have regarding a medical condition. Always remember to
- Ask your own doctor for medical advice.
- Names, brands, and dosage may differ between countries.
- When not feeling well, or experiencing side effects always contact your own doctor.
Cyberchondria
The truth is that when we’re sick, or worried about getting sick, the internet won’t help.
According to Wikipedia, cyberchondria is a mental disorder consisting in the desire to independently make a diagnosis based on the symptoms of diseases described on Internet sites.
Why you can't look for symptoms on the Internet
If diagnoses could be made simply from a textbook or an article on a website, we would all be doctors and treat ourselves. Nothing can replace the experience and knowledge of specially trained people. As in any field, in medicine there are unscrupulous specialists, differences of opinion, inaccurate diagnoses and incorrect test results.


Reviews
There are no reviews yet.